Plastics, more importantly microplastics, clog our oceans. This phenomena in the ocean has been likened to smog around cities. These plastic particles are dangerous because they can absorb toxins, subsequently be consumed by zooplankton and invertebrates, and bioaccumluate up the food web to fish that are consumed by humans. A study in Nature found that 25 percent of seafood sold contains microplastics! There has been a recent awareness of the unseen harm that exists when plastic pollution in the ocean degrades into microplastics. A report in Environmental Research Letters estimated that “accumulated number of micro plastic particles… ranges from 15 to 51 trillion particles, weighing between 93 and 236 thousand metric tons.” That is cray cray. Despite a better awareness of the impact of microplastics on marine ecology, we still have a poor spatial understanding of microplastics in the ocean. The presence and density of microplastics is determined by trawling the ocean (i.e., researchers go out with a net and physically count the pieces of plastic they pick up). As you can imagine, this is not very effective.

This could soon change. A team of Harvard undergraduates developed a tool, Plastiback, that both identifies the presence of microplastics, and degrades them. Plastiback is an ingenuous floating bioreactor containing genetically modified E. coli bacteria. The bacteria are used to identify and degrade a specific type of microplastic called polyethylene terephthalate (PET) – commonly used in water bottles and fishing equipment. So how does Plastiback work? The genetically engineered E. coli secrete an enzyme that breaks down PET into terephthalic acid and ethylene glycol. A fluorescent protein glows green when the PET-degrading enzyme is expressed identifying the presence of microplastics. Because the byproducts of microplastic are toxic, the system goes a step further and the terephthalic acid is metabolized by a second type of bacteria, D. tsuruhatensis sp. nov. producing electrons used to generate a small amount of voltage. The voltage is used to quantify how much PET was broken down by Plastiback, so the system both identifies the presence and quantifies the amount of microplastics broken down.
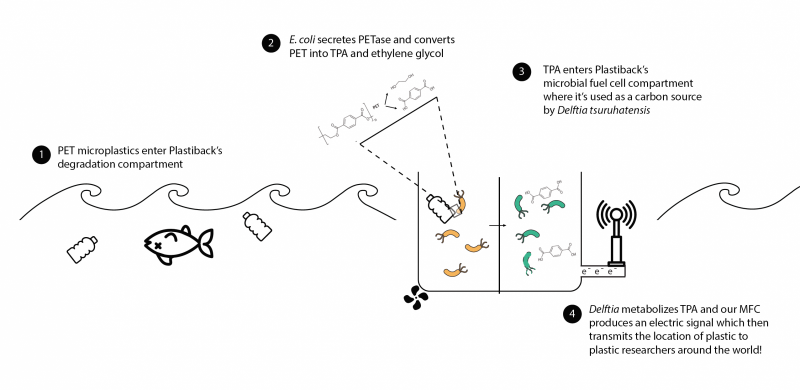
This is an ingenious developent, and if scalable could be utilized to provide a better sense of the spatial distribution of microplastics while also working on reducing the concentrations of microplastics in the ocean. So lets give a big shoutout to the team which included students Gabriela Berner, Will Cho, Rebekah Chun, and Mofeyifoluwa Edun. They were mentored by Neel Joshi, Associate Professor of Chemical and Biological Engineering, and graduate students Pichet Praveschotinunt, David Lips, Kevin Hof, and Anita Chandrahas.
You can learn more about Plastiback at their website.