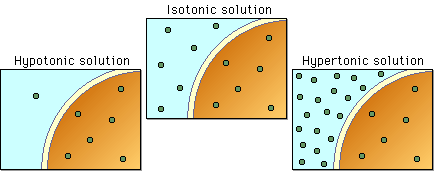
One of the challenges inherent in a marine lifestyle is in maintaining an internal balance against external osmotic pressures. Cell membranes are permeable to water, and water tends to flow from areas of low ion concentration to areas of high ion concentration (which is called ‘osmosis’). Though the cell is incredibly complex, from an osmotic perspective it is basically a small sack of water with some ions in it. If cells aren’t isosmotic (i.e. containing the same concentrations of ions) to the surrounding environment, then water will flow across a cell membrane. Depending on the relative ionic concentration of the cell to the environment, water may flow either into or out of a cell. Either way, this water flow is bad for the organism and may result in cells shriveling up or bursting.
One of the challenges inherent in a marine lifestyle is in maintaining an internal balance against external osmotic pressures. Cell membranes are permeable to water, and water tends to flow from areas of low ion concentration to areas of high ion concentration (which is called ‘osmosis’). Though the cell is incredibly complex, from an osmotic perspective it is basically a small sack of water with some ions in it. If cells aren’t isosmotic (i.e. containing the same concentrations of ions) to the surrounding environment, then water will flow across a cell membrane. Depending on the relative ionic concentration of the cell to the environment, water may flow either into or out of a cell. Either way, this water flow is bad for the organism and may result in cells shriveling up or bursting.