 One of the challenges inherent in a marine lifestyle is in maintaining an internal balance against external osmotic pressures. Cell membranes are permeable to water, and water tends to flow from areas of low ion concentration to areas of high ion concentration (which is called ‘osmosis’). Though the cell is incredibly complex, from an osmotic perspective it is basically a small sack of water with some ions in it. If cells aren’t isosmotic (i.e. containing the same concentrations of ions) to the surrounding environment, then water will flow across a cell membrane. Depending on the relative ionic concentration of the cell to the environment, water may flow either into or out of a cell. Either way, this water flow is bad for the organism and may result in cells shriveling up or bursting.
One of the challenges inherent in a marine lifestyle is in maintaining an internal balance against external osmotic pressures. Cell membranes are permeable to water, and water tends to flow from areas of low ion concentration to areas of high ion concentration (which is called ‘osmosis’). Though the cell is incredibly complex, from an osmotic perspective it is basically a small sack of water with some ions in it. If cells aren’t isosmotic (i.e. containing the same concentrations of ions) to the surrounding environment, then water will flow across a cell membrane. Depending on the relative ionic concentration of the cell to the environment, water may flow either into or out of a cell. Either way, this water flow is bad for the organism and may result in cells shriveling up or bursting.
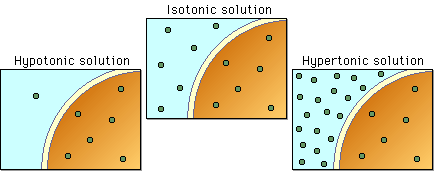
Different organisms solve this problem in a variety of different ways.
Hagfish and many marine invertebrates are osmoconformers and ion conformers. They simply keep their body fluids isosmotic with seawater by using the same ions found in seawater. If there is no osmotic difference between the seawater and their body fluids, then water won’t flow one way or the other.
Most teleost fish are osmoregulators and ion regulators. They keep their body fluids osmotically distinct from seawater and actively work to counter the effects of osmosis. Since there are fewer ions in fish body fluid than there are in seawater, fish are constantly losing water. To deal with this, marine fish are “drinking” seawater almost constantly. Since they only want the water and not the associated salt, they have special cells called chloride pumps that remove the extra salt.
In contrast, sharks (along with amphibians and coelocanths) are osmoconformers and ion regulators. Their body fluids are almost the same concentration of ions as seawater, but they use different ions. Sharks do have to deal with a slight influx of salt, which is excreted by a rectal gland.
One of the ions that sharks use is urea. Urea is relatively easy to produce (most organisms already make it in some form, they just excrete it), and works just fine as an ion from an osmotic perspective. The main downside is that urea is that it has a destabilizing effect on many enzymes, which is countered with the use of another ion: tri-methyl amine oxide (TMAO). After a shark dies, the urea in their body fluids converts into the foul-smelling and toxic ammonia.
Sharks are often thought of as “primitive” organisms, but they have a complex and effective method for living in salt water. Sharks’ use of a waste product to maintain osmotic balance is yet another amazing thing about these animals.
~WhySharksMatter
Burger, J., & Hess, W. (1960). Function of the Rectal Gland in the Spiny Dogfish Science, 131 (3401), 670-671 DOI: 10.1126/science.131.3401.670
Foskett JK, & Scheffey C (1982). The chloride cell: definitive identification as the salt-secretory cell in teleosts. Science (New York, N.Y.), 215 (4529), 164-6 PMID: 7053566
SMITH., H. (1936). THE RETENTION AND PHYSIOLOGICAL ROLE OF UREA IN THE ELASMOBRANCHII Biological Reviews, 11 (1), 49-82 DOI: 10.1111/j.1469-185X.1936.tb00497.x

Those photographic diagrams are misleading. Iso- hypo- and hypertonic solutions are named so because of a comparison with a different solution, not because the ion concentrations are at any specific level. Highly concentrated solutions can still be considered hypotonic and solutions with a low concentration can be hypertonic. The comparison across solutions is something that a lot of science texts leave out or de-emphasize, but it’s extremely important when you’re talking about osmoregulation.
Sam, I’m not sure that I understand your point.
Yes, those terms are all relative, but I don’t think that makes the diagram misleading. Also, the diagram is far from crucial to the point of this post.
It’s a bugbear I have. Too many classes discuss osmoregulation but leave it up to the student to realize that terms such as “hypertonic” et al are relative. My issue with the diagram is that it doesn’t specify which solution is hyper- iso- or hypotonic. A lot of texts do that and I’ve explained that the terms are used for comparison more times to other students than I care to count.
I understand that this is a small point of your post, but those diagrams tend to cause a lot of confusion.
David – have you looked into the relationship between TMAO, Urea and depth? It’s fascinating literature. AT the surface, the urea/TMAO ratio is a pretty standard 3:1 across most animal groups, regardless of absolute urea concentration. But as you go deeper, the effects of urea on protein folding and enzyme kinetics get more and more pronounced and the animal needs more and more TMAO to offset it. The relationship between urea/TMAO ratio and preferred depth range is incredibly tight. Paul Yancey has done a lot of great work in this area, but I think there’s a lot more yet to do, especially for TMAO, which has been underemphasised up to this point, IMHO.
I didn’t know that, Al. Thanks for sharing.
@sam’s reply post, the diagram is looking at the tonicity relative to the cell pictured and clearly demonstrates the the concept. Inside the cell, the concentration remains constant and the external environment is changing to show what each situation would look like